Ethosuximide is an succinimide based anticonvulsant commonly used for absence (petit mal) seizures in both adults and children. Ethosuximide has been associated with rare instances of serum enzyme elevations during treatment, but has not been linked to cases of clinically apparent liver injury with jaundice.
Mechanism of action
The mechanism by which ethosuximide affects neuronal excitability includes block of T-type calcium channels, and may include effects of the drug on other classes of ion channel. The primary finding that ethosuximide is a T-type calcium channel blocker gained widespread support, but initial attempts to replicate the finding were inconsistent. Subsequent experiments on recombinant T-type channels in cell lines demonstrated conclusively that ethosuximide blocks all T-type calcium channel isoforms.[citation needed] Significant T-type calcium channel density occurs in dendrites of neurons, and recordings from reduced preparations that strip away this dendritic source of T-type calcium channels may have contributed to reports of ethosuximide ineffectiveness.
In March 1989, Coulter, Huguenard and Prince showed that ethosuximide and dimethadione, both effective anti-absence agents, reduced low-threshold Ca2+currents in T-type calcium channels in freshly removed thalamic neurons.[5] In June of that same year, they also found the mechanism of this reduction to be voltage-dependent, using acutely dissociated neurons of rats and guinea pigs; it was also noted that valproic acid, which is also used in absence seizures, did not do that.[6] The next year, they showed that anticonvulsant succinimides did this and that the pro-convulsant ones did not.[7] The first part was supported by Kostyuk et al. in 1992, who reported a substantial reduction in current in dorsal root ganglia at concentrations ranging from 7 µmol/L to 1 mmol/L.[8]
That same year, however, Herrington and Lingle found no such effect at concentrations of up to 2.5 mmol/L.[9] The year after, a study conducted on human neocortical cells removed during surgery for intractable epilepsy, the first to use human tissue, found that ethosuximide had no effect on Ca2+ currents at the concentrations typically needed for a therapeutic effect.[10]
In 1998, Slobodan M. Todorovic and Christopher J. Lingle of Washington University reported a 100% block of T-type current in dorsal root ganglia at 23.7 ± 0.5 mmol/L, far higher than Kostyuk reported.[11] That same year, Leresche et al. reported that ethosuximide had no effect on T-type currents, but did decrease noninactivating Na+ current by 60% and the Ca2+-activated K+ currents by 39.1 ± 6.4% in rat and cat thalamocortical cells. It was concluded that the decrease in Na+ current is responsible for the anti-absence properties.[12]
In the introduction of a paper published in 2001, Dr. Juan Carlos Gomora and colleagues at the University of Virginia in Charlottesville pointed out that past studies were often done in isolated neurons that had lost most of their T-type channels.[13] Using cloned α1G, α1H, and α1I T-type calcium channels, Gomora's team found that ethosuximide blocked the channels with an IC50 of 12 ± 2 mmol/L and that of N-desmethylmethsuximide (the active metabolite of mesuximide) is 1.95 ± 0.19 mmol/L for α1G, 1.82 ± 0.16 mmol/L for α1I, and 3.0 ± 0.3 mmol/L for α1H. It was suggested that the blockade of open channels is facilitated by ethosuximide's physically plugging the channels when current flows inward.
Stereochemistry
Ethosuximide is a chiral drug with a stereocenter. Therapeutically, the racemate, the 1: 1 mixture of ( S ) and ( R ) - isomers used.[8]
| Enantiomers of ethosuximide | |
|---|---|
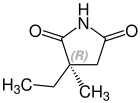 CAS-Nummer: 39122-20-8 |  CAS-Nummer: 39122-19-5 |

No comments:
Post a Comment